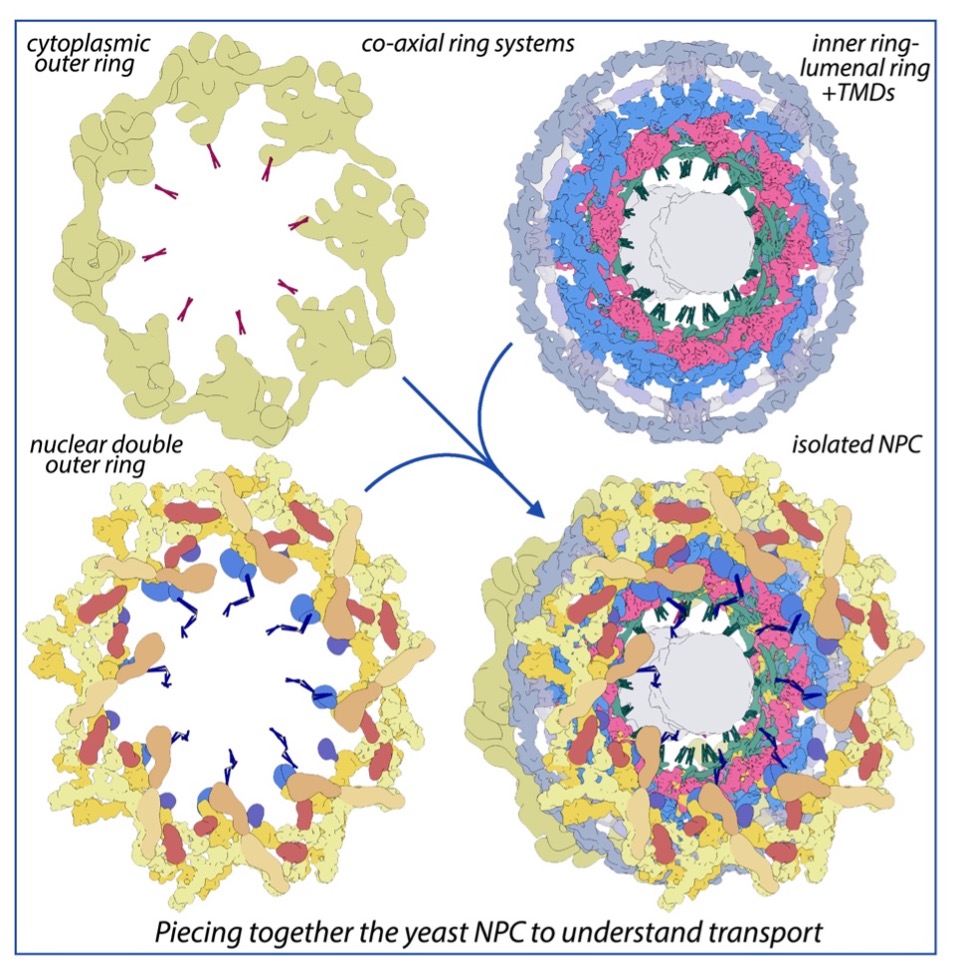
Nuclear pore complexes (NPCs) are the sole mediators of trafficking between the nucleus and cytoplasm. Sitting in the nuclear envelope, they are giant macromolecular assemblies that selective transport macromolecules bidirectionally to and from the nucleoplasm. In this latest update of our structural and functional mapping of the nuclear pore complex, we provide a composite multiscale structure of the yeast NPC, based on improved 3D density maps from cryogenic electron microscopy and artificial intelligence (AI) driven AlphaFold2 models of the individual nucleoporins comprising this structure. We resolved a host of flexible and dynamic connectors that tie together the entire core scaffold, providing a strong yet yielding architecture to the NPC. We have also begun to resolve the bundle of equatorial transmembrane regions that connect the NPC’s lumenal ring to the inner ring of the core scaffold, and so anchor the entire complex within the pore membrane. The organization of the nuclear double outer ring reveals an architecture that may be shared with ancestral NPCs. Additional connections between the core scaffold and the central transporter – the heart of the NPC’s transport machinery – suggest that under certain conditions, a degree of local organization is present at the periphery of this transport machinery. These connectors may couple conformational changes in the scaffold to the central transporter to modulate transport and suggest different regions of the central transporter may mediate distinct types of trafficking.
To read more, the paper can be freely accessed by clicking below:
You can subscribe to the latest NCDIR’s Rout lab news via twitter: