A multi-lab team has worked with the NCDIR to make breakthrough developments in the realm of cancer biomarkers. The team engineering nanobodies – small, robust variants of monoclonal antibodies – for use in ultrasensitive and cost-effective pan-cancer biomarker assays. The research spotlighted LINE-1 as a uniquely specific marker for cancer, which, unlike in normal tissues, is evident not only in tissue but also in blood, bolstering its potential as an early detection tool and a prognostic metric.
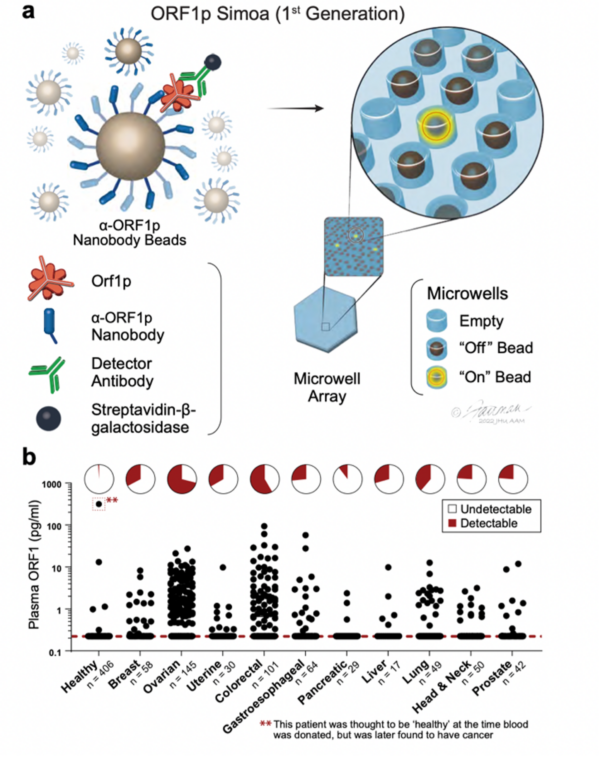
Highly Specific Detection of Carcinomas with the First-Generation ORF1p Simoa Assay. a, Schematic of single-molecule protein detection by Simoa; a first-generation assay is shown. Antibody/nanobody-coated magnetic beads, present in excess relative to target, capture single target ORF1p molecules; in the first-generation assay, beads are conjugated with α-ORF1p capture nanobody 5 (Nb5). Enzyme-labeled α-ORF1p detection reagent (here, an antibody, Ab6) is added, forming an “immunosandwich”; the beads are then loaded into microwells that each can hold at most one bead, and ORF1p molecules are then digitally detected using a fluorogenic substrate by counting “on” wells. b, First-generation ORF1p Simoa detects plasma ORF1p with high specificity across major carcinomas. Pie charts indicate percentage of samples with detectable levels; dashed red line, limit of detection.
Emphasizing collaboration, the study harnessed the dual expertise of NCDIR in producing specialized nanobodies and state-of-the-art mass spectrometry assays, and leverages a single-molecule-based detection technology known as SIMOA (developed by one of the team labs.
The test aims to identify the open reading frame 1 protein (ORF1p), a protein expressed by the mobile element LINE-1. High ORF1 expression is prevalent in numerous cancers, and signals a heightened risk of fatal cancer. Thus, for years, scientists have pursued a precise and sensitive method to detect ORF1p. The team characterized their research as an initial trial that exceeded their expectations, subsequently enhanced by a sequence of engineering refinements. The team utilized sophisticated immunohistochemistry stains, revealing a more widespread presence of LINE-1 in colon and gastroesophageal cancers. To counter the challenge posed by the extremely low LINE-1 levels in blood, they designed a cutting-edge SIMOA assay, using a combination of nanobodies and antibodies.
This assay was not only highly specific but also flagged an individual who was initially deemed healthy but later diagnosed with advanced prostate cancer. The team further refined their assays, producing 2nd and 3rd generation versions using custom nanobodies and antibodies, enhancing detection rates exponentially. These optimized assays demonstrated commendable accuracy in detecting early-stage lung and ovarian cancers and showcased their prognostic value, especially in the context of gastroesophageal and colon cancers. Notably, these assays can detect therapy response in gastroesophageal cancer earlier than other metrics. Plus, they stand out for their potential affordability, rapid results, and minimal blood requirement., making them excellent candidate assays for widespread clinical use.
Related publication:
Taylor MS, Connie W, Fridy PC, Zhang SJ, Senussi Y, Wolters JC, Cheng WC, Heaps J, Miller BD, Mori K, Cohen L, Jiang H, Molloy KR, Norden BL, Chait BT, Goggins M, Bhan I, Franses JW, Yang X, Taplin ME, Wang X, Christiani DC, Johnson BE, Meyerson M, Uppaluri R, Egloff AM, Denault EN, Spring LM, Wang TL, Shih IM, Jung E, Arora KS, Zukerberg LR, Yilmaz OH, Chi G, Matulonis UA, Song Y, Nieman L, Parikh AR, Strickland M, Corcoran RB, Mustelin T, Eng G, Yilmaz ÃH, Skates SJ, Rueda BR, Drapkin R, Klempner SJ, Deshpande V, Ting DT, Rout MP, LaCava J, Walt DR, Burns KH. “Ultrasensitive detection of circulating LINE-1 ORF1p as a specific multi-cancer biomarker.” Cancer Discov. 2023 Sep 12. doi: 10.1158/2159-8290.CD-23-0313. Online ahead of print. PMID: 37698949
The paper can be freely accessed here: https://aacrjournals.org/cancerdiscovery/article/doi/10.1158/2159-8290.CD-23-0313/729035/Ultrasensitive-detection-of-circulating-LINE-1